Wikisage, the free encyclopedia of the second generation, is digital heritage
Diacomit
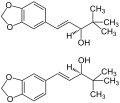
Diacomit ®contains 250 mg stiripentol[1][2]
In December 2001 the European Medicines Agency (EMA) granted stiripentol orphan drug status (designation number EU/3/01/071) for the treatment of severe myoclonic epilepsy of infancy (Dravet's syndrome). On 4 January 2007, the EMA granted the drug a marketing authorisation that is valid throughout the European Union
Dravet's syndrome does not exist data for combined therapy both valproic acid or clobazam[4]
ATC
http://www.whocc.no/atc_ddd_index/?code=N03AX17
| References: |
|