Wikisage, the free encyclopedia of the second generation, is digital heritage
Lennox-Gastaut syndrome: Difference between revisions
m (→Clobazam: FIG) |
(spect todo) |
||
| Line 11: | Line 11: | ||
==Clobazam== | ==Clobazam== | ||
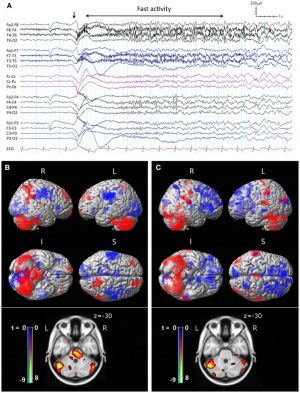
[[File:Fneur-05-00225-g001.jpg|thumb| | [[File:Fneur-05-00225-g001.jpg|thumb|Archer et al. Ictal EEG features and peri-ictal SPECT of tonic seizures in LGS. Frontiers in Neurology 5 2014]] | ||
[[Clobazam]] was approved by FDA on 2011 as adjuntive treatment of seisures associated with LGS in patients 2 year and older | [[Clobazam]] was approved by FDA on 2011 as adjuntive treatment of seisures associated with LGS in patients 2 year and older | ||
<ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4467745/pdf/tcrm-11-905.pdf Comprehensive overview: efficacy, tolerability, and cost-effectiveness of clobazam in Lennox-Gastaut syndrome]</ref> | <ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4467745/pdf/tcrm-11-905.pdf Comprehensive overview: efficacy, tolerability, and cost-effectiveness of clobazam in Lennox-Gastaut syndrome]</ref> | ||
Revision as of 22:18, 31 January 2016
Lennox–Gastaut syndrome (LGS[1]) is a difficult-to-treat form of childhood-onset epilepsy that most often appears between the second and sixth year of life. LGS is characterized by a triad of signs including frequent seizures of multiple types, an abnormal EEG pattern of less than 2.5 Hz slow spike wave activity,[2]
LGS children with a history of perinatal hypoxia or other perinatal event have earlier age of onset of seizures[3]
Links
Endoscopic epilepsy surgery: Emergence of a new procedure
Rufinamide
Clobazam
Clobazam was approved by FDA on 2011 as adjuntive treatment of seisures associated with LGS in patients 2 year and older [4]
stable dosage of clobazam for LGS are associated Epilepsia 55: 558(2014)