Wikisage, the free encyclopedia of the second generation, is digital heritage
Belzutifan: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
==ATC== | ==ATC== | ||
<gallery>File:Belzutifan.png</gallery> | <gallery>File:Belzutifan.png</gallery> | ||
Retinal angioma associated with cerebellar angioma and sometimes angioma in other organs<ref>Dictionary of Medical eponyms/ref> | Retinal angioma associated with cerebellar angioma and sometimes angioma in other organs<ref>Dictionary of Medical eponyms</ref> | ||
{{wikidata|Q27456641}} | {{wikidata|Q27456641}} | ||
Revision as of 23:24, 13 August 2021
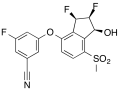
Belzutifan is a drug being approved for the treatment of renal cell carcinoma associated with von Hippel-Lindau disease.
Belzutifan is an inhibitor of hypoxia-inducible factor 2 alpha (HIF-2α).
Belzutifan is the first drug to be awarded an "innovation passport" by the UK Medicines and Healthcare products Regulatory Agency (MHRA)[1]
ATC
Retinal angioma associated with cerebellar angioma and sometimes angioma in other organs[2]
- ↑ https://en.wikipedia.org/wiki/Medicines_and_Healthcare_products_Regulatory_Agency
- ↑ Dictionary of Medical eponyms