Wikisage, the free encyclopedia of the second generation, is digital heritage
Rufinamide: Difference between revisions
Jump to navigation
Jump to search
m (→Weblinks) |
No edit summary |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
Otsuka et al. (2014) state rufinamide as an efficacious and well tolerated [[Antiepileptic drug|AED]] | Otsuka et al. (2014) state rufinamide as an efficacious and well tolerated [[Antiepileptic drug|AED]] | ||
<ref>[http://www.epires-journal.com/article/S0920-1211(14)00228-9/pdf Rufinamide as an adjunctive therapy for Lennox-Gastaut syndrome: a randomized double-blind placebo-controlled trial in Japan]</ref>(cf. Alssad et Coren 2014) <ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4256616/pdf/bcp0078-1264.pdf Exposure to rufinamide and risks of CNS adverse events in drug-resistant epilepsy: a meta-analysis of randomized, placebo-controlled trials] </ref> | <ref>[http://www.epires-journal.com/article/S0920-1211(14)00228-9/pdf Rufinamide as an adjunctive therapy for Lennox-Gastaut syndrome: a randomized double-blind placebo-controlled trial in Japan]</ref>(cf. Alssad et Coren 2014) <ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4256616/pdf/bcp0078-1264.pdf Exposure to rufinamide and risks of CNS adverse events in drug-resistant epilepsy: a meta-analysis of randomized, placebo-controlled trials] </ref> | ||
<gallery>File:Under construction icon.png</gallery> | |||
<small>"Rufinamide received the status of orphan drug for [[epilepsy]] in 2004 in Europe"</small> | <small>"Rufinamide received the status of orphan drug for [[epilepsy]] in 2004 in Europe"</small> | ||
<ref>[http://onlinelibrary.wiley.com/doi/10.1111/epi.12689/epdf Safety and retention rate of rufinamide in 300 patients: a single pediatric epilepsy center experience]</ref> | <ref>[http://onlinelibrary.wiley.com/doi/10.1111/epi.12689/epdf Safety and retention rate of rufinamide in 300 patients: a single pediatric epilepsy center experience]</ref> | ||
It is an inductor of [[Cytochrome P450|CYP 450]] | |||
==Weblinks== | ==Weblinks== | ||
| Line 12: | Line 12: | ||
[http://www.pharmawiki.ch/wiki/index.php?wiki=Rufinamid Pharmawiki:Rufinamid] | [http://www.pharmawiki.ch/wiki/index.php?wiki=Rufinamid Pharmawiki:Rufinamid] | ||
==ATC== | ==[[ATC]]== | ||
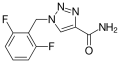
<gallery>File:Rufinamid.svg.png</gallery> | |||
https://www.whocc.no/atc_ddd_index/?code=N03AF03 | https://www.whocc.no/atc_ddd_index/?code=N03AF03 | ||
{{refs}} | |||
{{Wikidata|Q408565}} | {{Wikidata|Q408565}} | ||
[[Category:Anticonvulsants]] | [[Category:Anticonvulsants]] | ||
Latest revision as of 20:41, 20 March 2023
Lennox-Gastaut is a low prevalence epileptic syndrome (1:10000), and Cochrane Epilepsy Group concluded that treatment remain unconclusive. Otsuka et al. (2014) state rufinamide as an efficacious and well tolerated AED [1](cf. Alssad et Coren 2014) [2]
"Rufinamide received the status of orphan drug for epilepsy in 2004 in Europe" [3]
It is an inductor of CYP 450
Weblinks
ATC
https://www.whocc.no/atc_ddd_index/?code=N03AF03