Wikisage, the free encyclopedia of the second generation, is digital heritage
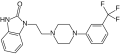
Flibanserin
flibaserin is a new FDA aproved drug, it was formerly developed in Europe by Boeringer Ingelheim latter in US by Sprout Pharmaceutical due it refusal by Health Authority in 2013[1]Flibanserin is a post-synaptic 5-hydroxytryptamine (5-HT) 1A receptor agonist and 5-HT 2A antagonist that has been evaluated for indications of major depressive disorder (MDD) and for the treatment of HSDD in premenopausal women.[2]
Links
Evaluation of Flibanserin Science and Advocacy at the FDA
Why are there no FDA-approved treatments for female sexual dysfunction?
| References: |