Wikisage, the free encyclopedia of the second generation, is digital heritage
Flibanserin: Difference between revisions
Jump to navigation
Jump to search
(→Links: hide) |
(Hypoactive sexual desire disorder) |
||
| Line 1: | Line 1: | ||
flibaserin is a new FDA aproved drug, it was formerly developed in Europe by Boeringer Ingelheim latter in US by Sprout Pharmaceutical due it refusal by Health Authority in 2013<ref>http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ReproductiveHealthDrugsAdvisoryCommittee/UCM215437.pdf</ref>Flibanserin is a post-synaptic 5-hydroxytryptamine (5-HT) 1A receptor agonist and 5-HT 2A antagonist that has been evaluated for indications of major depressive disorder (MDD) and for the | flibaserin is a new FDA aproved drug, it was formerly developed in Europe by Boeringer Ingelheim latter in US by Sprout Pharmaceutical due it refusal by Health Authority in 2013<ref>http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ReproductiveHealthDrugsAdvisoryCommittee/UCM215437.pdf</ref>Flibanserin is a post-synaptic 5-hydroxytryptamine (5-HT) 1A receptor agonist and 5-HT 2A antagonist that has been evaluated for indications of major depressive disorder (MDD) and for the | ||
treatment of HSDD in premenopausal women.<ref>http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM449088.pdf</ref> | treatment of [[Hypoactive sexual desire disorder|HSDD]] in premenopausal women.<ref>http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM449088.pdf</ref> | ||
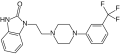
<gallery>File:flibanserin-structural.svg.png</gallery> | <gallery>File:flibanserin-structural.svg.png</gallery> | ||
Revision as of 01:24, 16 September 2015
flibaserin is a new FDA aproved drug, it was formerly developed in Europe by Boeringer Ingelheim latter in US by Sprout Pharmaceutical due it refusal by Health Authority in 2013[1]Flibanserin is a post-synaptic 5-hydroxytryptamine (5-HT) 1A receptor agonist and 5-HT 2A antagonist that has been evaluated for indications of major depressive disorder (MDD) and for the treatment of HSDD in premenopausal women.[2]
Links
Evaluation of Flibanserin Science and Advocacy at the FDA
Why are there no FDA-approved treatments for female sexual dysfunction?