Wikisage, the free encyclopedia of the second generation, is digital heritage
Flibanserin: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
(txt) |
||
| Line 1: | Line 1: | ||
fibraserin is a new FDA aproved drug. | fibraserin is a new FDA aproved drug. | ||
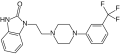
Former developed in nEurope by Boeringer I latter in US by Sprout Pharmaceutical due it refusal by Health Authory in 2013<ref>http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ReproductiveHealthDrugsAdvisoryCommittee/UCM215437.pdf</ref><ref>http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM449088.pdf</ref> | Former developed in nEurope by Boeringer I latter in US by Sprout Pharmaceutical due it refusal by Health Authory in 2013<ref>http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ReproductiveHealthDrugsAdvisoryCommittee/UCM215437.pdf</ref>Flibanserin is a post-synaptic 5-hydroxytryptamine (5-HT) 1A receptor agonist and 5-HT 2A antagonist that has been evaluated for indications of major depressive disorder (MDD) and for the | ||
treatment of HSDD in premenopausal women.<ref>http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM449088.pdf</ref> | |||
<gallery>File:flibanserin-structural.svg.png</gallery> | <gallery>File:flibanserin-structural.svg.png</gallery> | ||
<references/> | <references/> | ||
Revision as of 18:01, 19 August 2015
fibraserin is a new FDA aproved drug. Former developed in nEurope by Boeringer I latter in US by Sprout Pharmaceutical due it refusal by Health Authory in 2013[1]Flibanserin is a post-synaptic 5-hydroxytryptamine (5-HT) 1A receptor agonist and 5-HT 2A antagonist that has been evaluated for indications of major depressive disorder (MDD) and for the treatment of HSDD in premenopausal women.[2]