Wikisage, the free encyclopedia of the second generation, is digital heritage
Diacomit: Difference between revisions
Jump to navigation
Jump to search
mNo edit summary |
mNo edit summary |
||
| Line 1: | Line 1: | ||
Diacomit contains 250 mg stiripentol<ref>http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/000664/WC500036518.pdf</ref><ref>A new type of anticonvulsant, stiripentol. Pharmacological profile and neurochemical study. Arzneimittelforschung. 1984; 34(2):199-204.</ref> | Diacomit contains 250 mg stiripentol<ref>http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/000664/WC500036518.pdf</ref><ref>A new type of anticonvulsant, stiripentol. Pharmacological profile and neurochemical study. Arzneimittelforschung. 1984; 34(2):199-204.</ref> | ||
<ref>[http://onlinelibrary.wiley.com/doi/10.1111/j.1528-1167.2006.00497.x/pdf Stiripentol, a Putative Antiepileptic Drug, Enhances the Duration of Opening of GABA<sub>A</sub>-Receptor Channels]</ref> | |||
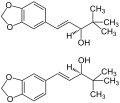
<gallery>File:(±)-Stiripentol Structural Formulae V.1.svg.png</gallery> | <gallery>File:(±)-Stiripentol Structural Formulae V.1.svg.png</gallery> | ||
In December 2001 the European Medicines Agency (EMA) granted stiripentol orphan drug status (designation number EU/3/01/071) for the treatment of severe myoclonic epilepsy of infancy (Dravet's syndrome). On 4 January 2007, the EMA granted the drug a marketing authorisation that is valid throughout the European Union | In December 2001 the European Medicines Agency (EMA) granted stiripentol orphan drug status (designation number EU/3/01/071) for the treatment of severe myoclonic epilepsy of infancy (Dravet's syndrome). On 4 January 2007, the EMA granted the drug a marketing authorisation that is valid throughout the European Union | ||
[[Dravet's syndrome]] | [[Dravet's syndrome]] does not exist data for combined therapy both valproic acid or clobazam<ref>Aras et al 2015</ref> | ||
does not exist data for combined therapy both valproic acid or | |||
==ATC== | ==ATC== | ||
http://www.whocc.no/atc_ddd_index/?code=N03AX17 | http://www.whocc.no/atc_ddd_index/?code=N03AX17 | ||
<references/> | <references/> | ||
Revision as of 19:16, 31 March 2016
Diacomit contains 250 mg stiripentol[1][2]
In December 2001 the European Medicines Agency (EMA) granted stiripentol orphan drug status (designation number EU/3/01/071) for the treatment of severe myoclonic epilepsy of infancy (Dravet's syndrome). On 4 January 2007, the EMA granted the drug a marketing authorisation that is valid throughout the European Union
Dravet's syndrome does not exist data for combined therapy both valproic acid or clobazam[4]
ATC
http://www.whocc.no/atc_ddd_index/?code=N03AX17
- ↑ http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/000664/WC500036518.pdf
- ↑ A new type of anticonvulsant, stiripentol. Pharmacological profile and neurochemical study. Arzneimittelforschung. 1984; 34(2):199-204.
- ↑ Stiripentol, a Putative Antiepileptic Drug, Enhances the Duration of Opening of GABAA-Receptor Channels
- ↑ Aras et al 2015