Wikisage, the free encyclopedia of the second generation, is digital heritage
Amifampridine: Difference between revisions
Jump to navigation
Jump to search
(→ATC) |
(→ATC) |
||
| Line 3: | Line 3: | ||
==ATC== | ==ATC== | ||
{| style="background:Ivory; color:black" border=1 cellspacing=1 cellpadding=5 | {| style="background:Ivory; color:black" border=1 cellspacing=1 cellpadding=5 | ||
|colspan=2| <center> | |colspan=2| <center> | ||
Revision as of 00:39, 7 May 2019
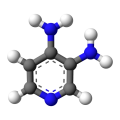
Amifampridine phosphate was granted an Orphan Drug designation by the FDA for MG in September 2016, shortly after receiving a Refusal to File letter from the FDA for its submission for the treatment of congenital myasthenic syndromes and Lambert Eaton myasthenic syndrome (LEMS [1])[2]
ATC
Links
https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-children-lambert-eaton-myasthenic-syndrome-rare-autoimmune-disorder Drug Trial Snapshot: Firdapse